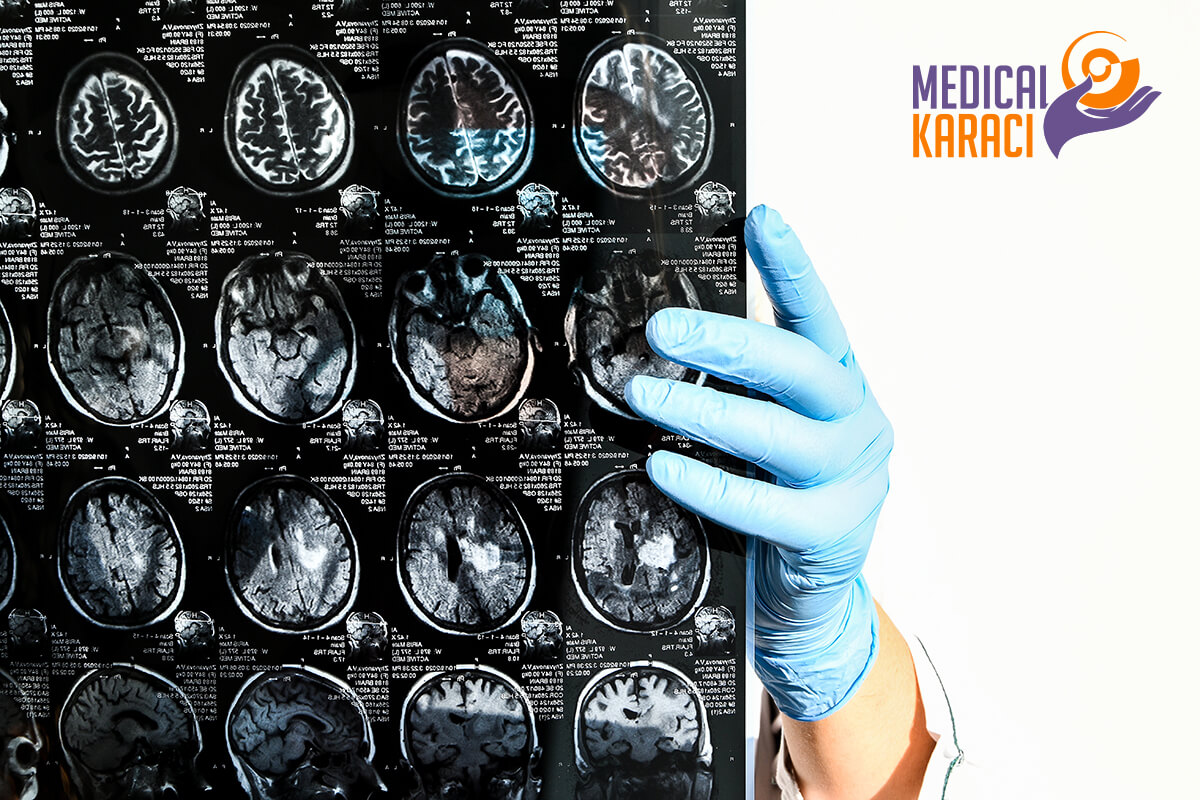
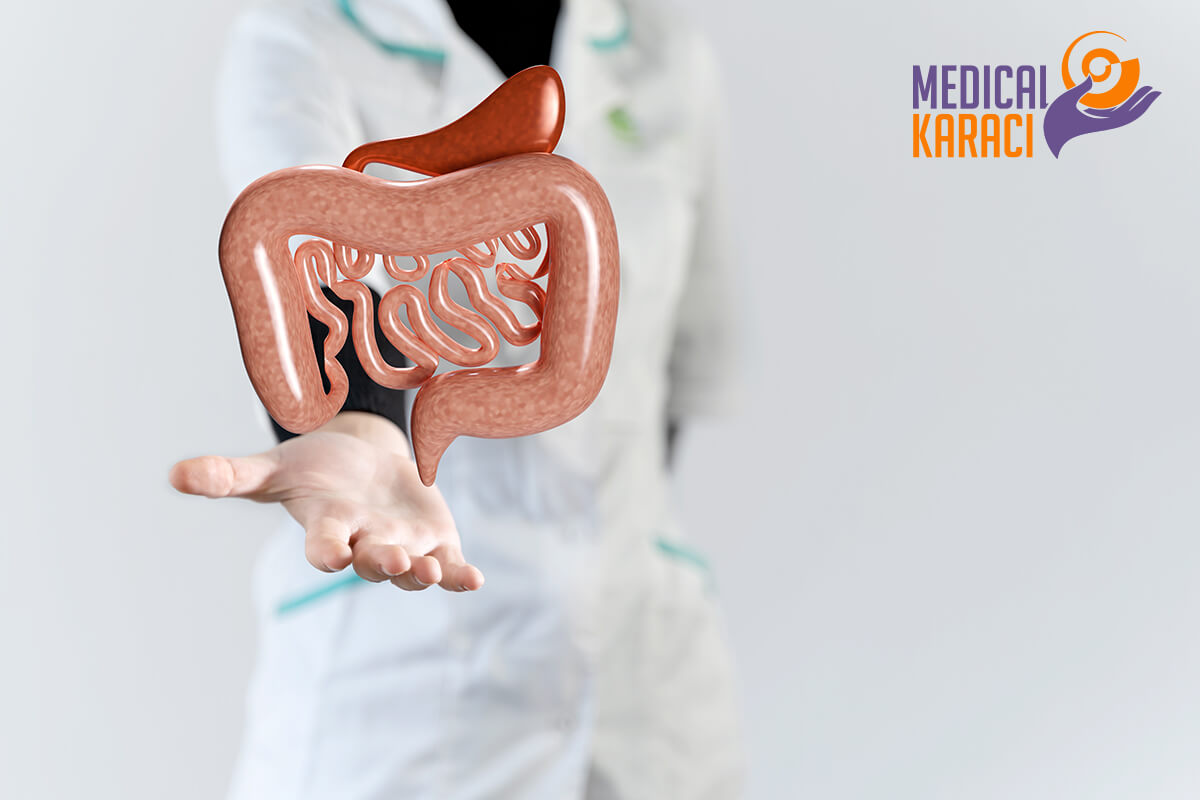
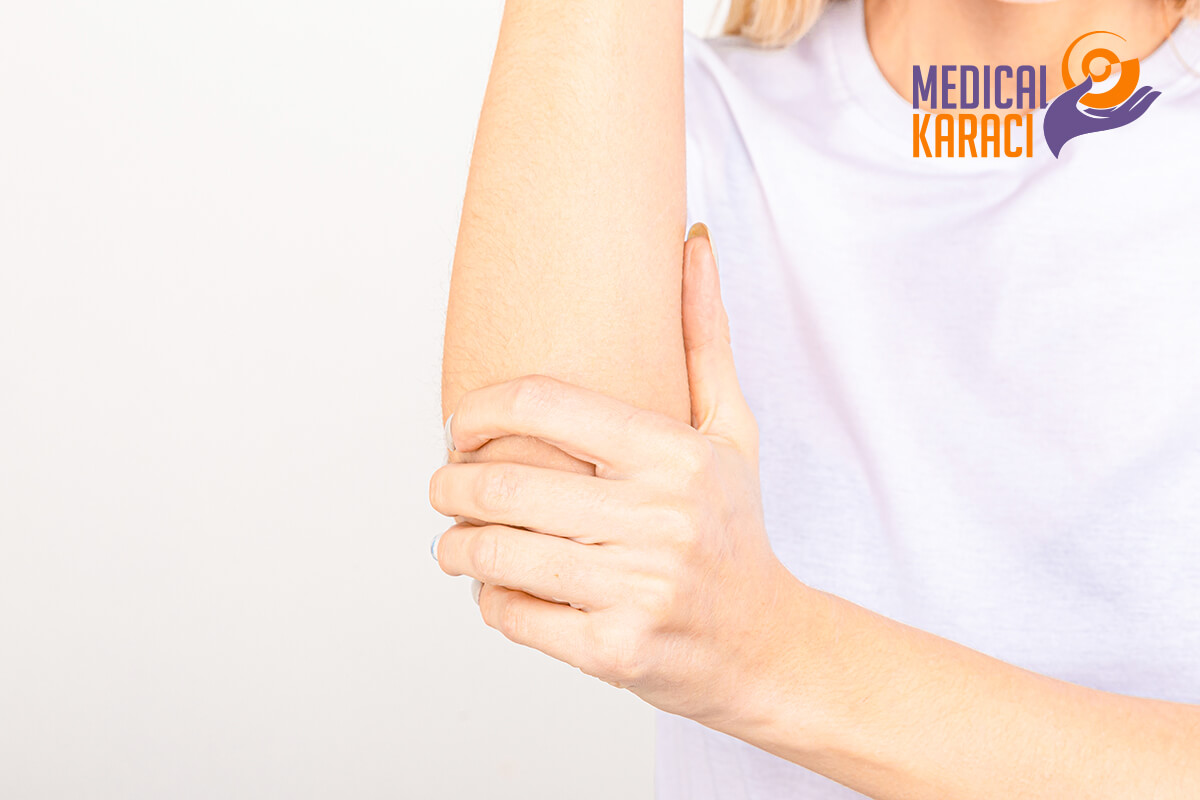
Regenerative medicine is a relatively new branch of medicine that develops methods to restore, replace or create damaged or diseased cells, tissues or organs. Regenerative medicine involves the generation and use of therapeutic stem cells, tissue engineering and the production of artificial organs.
Spanning a wide range of scientific disciplines, from molecular biology and genetics to immunology and biochemistry, regenerative medicine research generally focuses on the following key principles:
- Understanding the physiological mechanisms of stem cells.
- Knowledge of how cells grow and die.
- What makes stem cells turn into other cells?
- Learning more about the support structure between cells.
What makes regenerative medicine such an exciting field is that it has the potential to regenerate or replace any damaged tissue or organ in the body. This in turn leads to potentially providing a cure for many genetic and other diseases, and injuries.
What do regenerative medicine scientists want to know?
Older people already have some regenerative abilities. For example, the liver is able to grow back to its original size if part of it is lost due to injury or disease. Our skin is another part of our body that is able to renew and regenerate. Some tissues and organs do not have this ability, but scientists believe they could change this. To be able to do so, they need to answer a number of important questions:
- How is the cell potential determined? What unlocks it?
- How do some human tissues regenerate naturally (skin, blood cells and the lining of the digestive tract)?
- Why do parts of our bodies not regenerate (brain, heart) but retain a hidden ability to do so?
- How can some animals regenerate complex parts like a limb or a heart?
Answers to these questions are vital as they will provide insight into how stem cells work to repair or replace tissues and organs. Understanding the molecular and cellular mechanisms is essential for the development of regenerative medicine.
What are the applications of regenerative medicine?
There are three main ways in which regenerative medicine can be applied to patients.
Tissue Engineering
Illness or injury can lead to organ failure. The only solution for this is transplantation. The problem is that there are not enough donor organs and many people will lose their lives before they get their chance.
One of the goals of regenerative science is to be able to create new body parts from the patient's own cells and tissues. This would not only eliminate the demand for organs, but also the complications arising from their eventual rejection.
Immunomodulatory therapy
Embryonic stem cells, which are highly pluripotent - i.e. capable of becoming almost any type of cell are derived from the inner cell mass and are responsible for our growth. This means that stem cells have been able to divide and differentiate into other types of cells such as nerve cells, muscle cells and blood cells. When tissues or organs are damaged, they can potentially regenerate with these pluripotent stem cells. This process can reverse or prevent damage to vital organs.
Cell therapies
Many millions of adult stem cells are found in every person. The human body uses stem cells as one way to regenerate. Studies have shown that if adult stem cells are collected and then injected into the site of diseased or damaged tissue, tissue repair is possible under the right circumstances. These cells can be harvested from blood, fat, bone marrow, dental pulp, skeletal muscle and other sources. The umbilical cord is also a source of stem cells. Scientists are developing and refining their ability to prepare harvested stem cells to be injected into patients to repair diseased or damaged tissue.
Where do stem cells come from?
Researchers have discovered several sources of stem cells:
-
Embryonic stem cells
These stem cells come from embryos that are three to five days old. At this stage, the embryo is called a blastocyst and has about 150 cells. These are pluripotent stem cells, which means they can divide into more stem cells or they can turn into any type of cell in the body. This flexibility allows embryonic stem cells to be used to regenerate or repair diseased tissue or an organ.
-
Adult mesenchymal stem cells
These stem cells are found in small numbers in most adult tissues, such as bone marrow or fat. Compared to embryonic stem cells, adult stem cells have a more limited ability to turn into different cells of the body. Until recently, researchers believed that adult stem cells could only create similar types of cells. For example, researchers believe that stem cells residing in the bone marrow can only give rise to blood cells. The new evidence suggests that adult stem cells may be able to create different types of cells. For example, bone marrow stem cells may be able to create bone or cardiac muscle cells.
This research led to early-stage clinical trials to test its usefulness and safety in humans. For example, adult stem cells are currently being tested in people with neurological or heart disease.
-
Induced pluripotent stem cells
Adult stem cells that have been altered to have the properties of embryonic stem cells. Scientists have successfully transformed ordinary adult cells into stem cells using genetic reprogramming. By altering genes in adult cells, researchers can reprogram the cells to act similarly to embryonic stem cells. This new technique could allow researchers to use reprogrammed cells instead of embryonic stem cells and prevent the immune system from rejecting the new stem cells. However, scientists do not yet know whether using altered adult cells will cause adverse effects in humans.
-
Perinatal stem cells
Stem cells in the cord blood as well as in the amniotic fluid. These stem cells also have the ability to turn into specialized cells. Amniotic fluid fills the sac that surrounds and protects the developing fetus in the uterus. Researchers have identified stem cells in samples of amniotic fluid taken from pregnant women to test for abnormalities, a procedure called amniocentesis. More study of amniotic fluid stem cells is needed to understand their potential.
How are stem cells used in regenerative medicine?
With proper stimulation, many stem cells can take over the role of any cell type and can regenerate damaged tissue under the right conditions. This potential can save lives or repair wounds and tissue damage in people after illness or injury. Scientists see many possible uses for stem cells.
AUTISM
Autism is a neurological disorder characterised by impaired social interaction, verbal and non-verbal communication, and restricted and repetitive behaviour. Parents usually notice signs during the first two years of their child's life. These signs often develop gradually, although some children with autism reach their developmental milestones at a normal rate and then regress. Diagnostic criteria require that symptoms become evident in early childhood.
Autism affects information processing in the brain by changing the way nerve cells and their synapses connect and organize. How this happens is not well enough established.
Worldwide, autism affects more than 22 million people.
How can stem cells help?
Mesenchymal stem cell therapy may be useful in patients with autism. Implanted mesenchymal stem could differentiate into nerve cells, horizontal cells and microglia. With the medical control system, the differentiated neurons could be transferred to neurons that can secrete inhibitory neurotransmitter and regulate the function of the neurons that are paradoxically released. At the same time, some of the differentiated intermediate neurons can transfer the normal nerve impulse and initiate normal cell growth again.
The immune system is thought to play an important role in autism. Interactions between the immune system and the nervous system begin early during the embryonic stage and successful neurodevelopment depends on a balanced immune response. Currently, among stem cell classes, mesenchymal stem cells appear to have the greatest potential for cell therapy of autism spectrum disorders, possibly due to their immunomodulatory capacity. In addition, mesenchymal stem cells have a rapid self-renewal and proliferation rate, do not cause graft rejection upon transplantation, and are not tumorigenic. It has been suggested that they could function in the treatment of autism by secreting anti-inflammatory cytokines, stimulating growth factors, integrating into neural networks and restoring plasticity. Early clinical studies of transplantation of umbilical cord-derived mesenchymal stem cells have shown some effectiveness in the treatment of autism.
Treatment procedure:
Treatment consisted of 4 stem cell implantation procedures and self-activation of stem cells to repair motor neuron damage. The patient receives treatment to improve circulation to increase blood supply to these damaged nerves and nourish the neurons, along with daily rehabilitative exercise to promote recovery of motor function.
Expected result:
In autism, mesenchymal stem cells are expected to have a peripheral anti-inflammatory effect, with the potential to heal gut and silent autoimmune reactions. This method of treatment can make the patient's spirit and intelligence better. Eventually the disease can be brought under control.
MULTIPLE SCLEROSIS (MS)
What is multiple sclerosis?
Multiple sclerosis (MS) is a disease that affects nerve cells in the brain and spinal cord. In a healthy body, these nerve cells carry messages between the brain and the rest of the body, allowing us to move, balance, see, hear and feel. In MS, the body's own immune system attacks the nerve cells so that they cannot function properly.
Each nerve cell is encased in a protective sheath called myelin. If the myelin is damaged, the cell can no longer carry messages properly. This happens in MS. Depending on which nerves are damaged, patients can suffer a range of symptoms, often including problems walking and feeling, bladder and bowel problems, and fatigue. These symptoms occur for a time (relapse) and then improve (remission), often returning to normal as the body repairs the damaged myelin. Eventually, however, the nerve cells themselves are damaged and begin to degenerate until they stop working altogether. As the disease progresses, more nerve cells are affected, leading to increasing disability.
How is multiple sclerosis currently treated?
There is currently no cure for multiple sclerosis, but it is possible to treat the symptoms and reduce the number of relapses using medication, exercise and physiotherapy. These treatments aim to help patients manage symptoms or try to prevent nerve cell damage. They do not help to repair the damage once it has occurred. Researchers hope that stem cell therapies may provide new approaches that can both prevent damage and enable damage that has already occurred to be repaired.
How could stem cells help?
Mesenchymal stem cells are able to regulate autoimmune status, which can slow or even halt disease progression while restoring neural lesions that already exist. It is important to stress: biological treatment cannot be the only method used. To achieve the best result, it must be combined with the intake of certain amounts of medication and physical therapy.
- Preventing damage: It is possible to use certain types of stem cells to "reset" the immune system, known as immunomodulation. The goal is to prevent the immune system from attacking nerve cells or to reduce the amount of damage done.
- Repair: Stem cells may be able to help repair the damaged myelin sheath by "remyelinating" the nerves and allowing them to function properly again. This may prevent the nerves themselves from degenerating.
Treatment Plan:
Patients receive 2 courses of treatment. In the first course of stem cell treatment, the patient receives drugs to improve his brain environment, immunosuppressants that block the development of his disease. The patient will receive four implantations of mesenchymal stem cells via lumbar puncture. A series of drugs will also be used to help the stem cells grow. This is accompanied by daily rehabilitation to accelerate stem cell differentiation and recovery of bodily functions. In the second cycle of treatment, a drug is used to protect the stem cells and accelerate cell proliferation as rehabilitation continues.
A combination of two injection methods is used for patients diagnosed with multiple sclerosis: intravenous and lumbar puncture.
Intravenous injection
Efficiency
Based on clinical data and research, stem cells cross the blood-brain barrier when injected systemically.
Procedure
The intravenous delivery method is a very simple process. A catheter is inserted into the patient's vein. Usually, sedation is not required for this procedure. The whole process of intravenous injection takes no more than 45 minutes.
Lumbar puncture
Procedure
Lumbar puncture is also often called a spinal tap. It is a procedure used to access the cerebrospinal fluid of the brain and spinal cord. It helps deliver stem cells directly into the cerebral spinal fluid, bypassing the blood-brain barrier. It is the least invasive method of delivering stem cells directly into the central nervous system. The cerebrospinal fluid is used by the body to provide protection to the brain and spinal cord, limiting the possibility of injury to these areas. The body constantly produces cerebrospinal fluid and thus any fluid withdrawn is replaced naturally within a few hours.
Expected result:
Stem cell therapy in multiple sclerosis could potentially bring improvements in:
- motor function
- Sensitivity
- balance
- Coordination
- neuropathic pain
- Fatigue
- Sight
- The Shakes
- bladder and bowel control, etc.
It is important to understand that treatment is not a cure. Multiple sclerosis is a progressive condition and the goal of treatment is to temporarily reverse the symptoms of the disease to achieve a better quality of life.
Read more about multiple sclerosis
DIABETES
All cells in our body need energy. This energy is carried into the body as sugar (glucose) in the blood. Normally, blood sugar levels are controlled by the release of the hormone insulin. Insulin is produced by cells in the pancreas called beta cells, which are arranged in clusters along with other pancreatic cells. These clusters are called islets of Langerhans. There are approximately one million islets in a human pancreas.
There are several types of diabetes. What they all have in common is the problem of regulating normal blood sugar levels.
The most common types of diabetes:
- Type I diabetes occurs when the body's immune system damages and then destroys beta cells. This means that blood sugar levels remain high all the time, which can cause long-term damage to the body.
- Type II diabetes occurs when beta cells do not produce enough insulin or the insulin produced does not work properly (the body's cells become resistant to insulin).
When blood sugar levels rise, beta cells in the pancreas release insulin. Insulin tells cells in the body to take up glucose from the blood. In type I diabetes, the immune system destroys the beta cells. In type II diabetes, the cells do not take up enough glucose, either because they are insensitive to insulin or too little insulin is produced.
Patients with type I diabetes require daily blood testing and insulin injections.
How is diabetes treated?
Type II diabetes can often be at least partially controlled through a healthy diet and regular exercise; type I diabetes cannot. People with type I diabetes should test their blood sugar levels several times a day and administer insulin when needed (by injection or pump). Over time, high blood sugar levels can cause serious damage to:
- heart
- eyes
- blood vessels
- Kidney
- nerves
Injecting too much insulin can lead to blood sugar levels that are too low (hypoglycemia), which can be fatal.
Medical therapies for diabetes include replacing destroyed beta cells and restoring self-tolerance. Mesenchymal stem cells and their secretors, called "exosomes," may become an alternative therapy for patients with type I and type II diabetes. Transplants may allow the body to regain control of blood sugar levels so that insulin administration is no longer necessary.
How could exosomes derived from mesenchymal stem cells help?
Exosomes derived from intracellular compartments are about 40-100 nm in diameter. This method, which is called "nanoparticle treatment," involves intravenous administration of a nanoparticle (exosome) 4 times at one-week intervals.
Mesenchymal stem cell exosomes have been used in experimental and clinical settings to improve diabetes and reduce complications and published in many scientific studies. In the context of diabetes research, exosomes have been used to generate insulin-producing cells, counter autoimmunity, improve islet engraftment, and treat diabetic ulcers and limb ischemia.
Mesenchymal stem cells offer new treatment options for diabetes. Growing evidence of the potential therapeutic properties of stem cells justifies cautious optimism regarding the development of effective cell therapies for the treatment of diabetes. The hope is that after transplantation, mesenchymal stem cells and their secretions may provide protective molecules/factors to tissues and help restore some of the circuitry important to affected cells and tissues.
Scientists have successfully used mesenchymal stem cells and their secretors to produce glucose-responsive cells that secrete insulin, such as beta cells. Clinical trials of these cells are ongoing.
This method has no side effects.
Read more about type 1 and type 2 diabetes
DIABETIC FOOT
Diabetes mellitus can be seen as one of the "epidemics" of the Western world. It contributes to severe morbidity and mortality due to target organ damage (neuropathy, vasculopathy, nephropathy, retinopathy). Diabetic foot is defined as destruction or infection of tissue in the foot of diabetic patients due to neurological damage and/or varying levels of peripheral vascular disease. Complications of diabetic foot are the most common cause of lower limb amputations.
Treatment Plan:
It has been shown that mesenchymal stem cells can be an effective therapy for diabetic foot.
Inclusion and acceptance criteria for stem cell therapy:
- Informed consent signed by the patient.
- Adult men or women between 18 and 81 years of age with type I or type II diabetes mellitus
- The patient has one diabetic foot ulcer on the treated limb. The size of the ulcer is no larger than 10 cm2.
- The diabetic foot ulcer is neuropathic: The patient is checked with a 5.27 mm Monofilament and has no sensation in at least 4 of 9 points in the foot.
- No endovascular or surgical interventions are planned.
- The patient's life is not in immediate danger.
Treatment procedure:
The patient receives 4 stem cell implantation procedures and self-activation of stem cells to repair damaged tissue.
Purpose of treatment:
The hope is that in mesenchymal stem cell transplantation, the injured cells can provide protective molecules/factors to the tissues and help, indirectly by cell integration and differentiation, to repair the tissues of the affected limbs.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Chronic obstructive pulmonary disease (COPD) is an important health problem with high and steadily increasing mortality, morbidity and prevalence worldwide. Chronic lung diseases such as cancer and chronic obstructive pulmonary disease (COPD) are among the most common causes of death in Europe. They cost the EU more than €100 billion a year. These diseases involve changes in the cells of the lungs. Apart from smoking, knowledge of risk factors, disease pathogenesis and clinical features, as well as phenotypes and treatments, is very scarce. Drugs currently used for treatment aim to meet the patient's oxygen needs, not to increase lung capacity or cure the disability. When the injury to lung cells is irreversible in COPD, new treatments for the condition are needed.
Treatment Plan:
Some stem cells contribute to the initial development of the lungs, while others help to repair and regenerate the lung throughout life.
Mesenchymal cell exosome is a new type of therapy and is important for lung repair, acting at the right time and on the right targets and replacing the specialized cells that make up the lungs that naturally die and need to be replaced.
Treatment procedure:
The patient receives 4 implants of an exosome from stem cells derived from the umbilical cord. Transplantation of stem cell-derived exosomes reduces lung inflammation and collagen deposition in the pulmonary fibrosis. Exosomes play an important role in the effectiveness of regenerative therapies. They include a variety of cytokines, chemokines and growth factors. These small molecules can modulate the lung microenvironment and parenchyma.
Purpose of treatment:
The hope is that after transplantation, mesenchymal stem cells and their secretions can provide protective molecules/factors to tissues and help, indirectly with cell integration and differentiation, to restore some of the circuits important to the affected tissues. So, the goal of the treatment is to improve the patient's breathing and improve their quality of life.
CROWN DISEASE
Crohn's disease and ulcerative colitis are chronic bowel diseases characterized by idiopathic inflammation of the gastrointestinal tract. The peak age of onset is 15-30 years, irrespective of gender.
Crohn's disease is caused by a combination of environmental, immune and bacterial factors in genetically susceptible individuals. This results in a chronic inflammatory disorder in which the body's immune system attacks the gastrointestinal tract, possibly targeting microbial antigens. While Crohn's disease is linked to the immune system, it does not appear to be an autoimmune disease. About half of the overall risk is related to genetics.
There are no drugs or surgical procedures that can cure Crohn's disease. Treatment options help with symptoms, maintain remission and prevent recurrence.
How can mesenchymal stem cells help?
The ability of mesenchymal stem cells to suppress immune responses may be important in the treatment of Crohn's disease. It has been suggested that they could function in disease treatment by secreting anti-inflammatory cytokines, stimulating growth factors and integrating into gastrointestinal regulation. Early clinical trials of transplantation of human umbilical cord-derived mesenchymal stem cells have shown some efficacy in the treatment of Crohn's disease.
The goal of treatment is to improve control of immune system attacks on the gastrointestinal system.
RHEUMATOID ARTHRITIS
Rheumatoid arthritis is a chronic and systemic disease that primarily attacks the synovial joints. It leads to joint destruction and functional disability. The disease is characterized by warm, swollen and painful joints most commonly of the wrist and hands. The disease can also affect other parts of the body, can cause low red blood cell count, inflammation around the lungs and inflammation around the heart. Symptoms often appear gradually over weeks and months.
Although the cause of rheumatoid arthritis is not clear, it is thought to involve a combination of genetic and environmental factors. The underlying mechanism involves the body's immune system attacking the joints. This leads to inflammation and thickening of the joint capsule. It also affects the underlying bone and cartilage.
Diagnosis is mostly based on symptoms. Radiographs and laboratory tests can support the diagnosis or rule out other diseases with similar symptoms.
Other diseases that can manifest in a similar way include:
- systemic lupus erythematosus
- psoriatic arthritis
- fibromyalgia
How can mesenchymal stem cells help?
Mesenchymal stem cells are cells of stromal origin that can exert profound immunosuppression by modulating T and B cell proliferation and differentiation, dendritic cell maturation, and NK activity. These immunoregulatory properties promote the possible use of these cells to modulate autoimmune responses and in the treatment of autoimmune diseases.
In addition to their well-documented self-renewal and multipotent differentiation properties, umbilical cord mesenchymal stem cells also possess immunoregulatory capacity.
Treatment procedure:
The patient receives 4 stem cell implantation treatments and self-activation of stem cells to repair synovial joints and damaged tissues.
In rheumatoid arthritis, mesenchymal stem cells are expected to have a peripheral anti-inflammatory effect, with the potential to heal gut and silent autoimmune reactions. This method of treatment may improve the condition of the patient's synovial joints and damaged tissues. Eventually, the disease can be brought under control.
TENNIS LAKE
Tennis elbow or lateral epicondylitis is a condition in which the outer part of the elbow becomes painful and tender in the lateral epicondyle. The forearm muscles and tendons become damaged from repetitive overuse. This results in pain and tenderness on the outside of the elbow.
Any activity, including playing tennis, that involves repetitive use of the extensor muscles of the forearm can cause acute or chronic tendonitis. The condition is common in carpenters and workers who swing a hammer or other tool with the forearm, and is similar to Golfer's elbow, which affects the medial epicondyle on the inside of the elbow. Continued activity after the onset of the condition and avoidance of compulsory rest can result in permanent onset of pain and be treatable only by surgery.
How can mesenchymal stem cells help?
During the healing process, stem cells are able to differentiate into specialized cells - muscle, tendon, cartilage, bone - to renew the injured elbow. They also cause other important cells to come to the site of the injury, which in turn increases blood flow and the release of growth factors needed for repair and regeneration.
Treatment procedure:
Four stem cell implantation and stem cell activation treatments for forearm muscle and tendon repair. In tennis elbow, mesenchymal stem cells have a peripheral anti-inflammatory effect, with the potential to regenerate damaged tissue.
SPINAL CORD INJURY
How can stem cells contribute to spinal cord regeneration?
Studies have shown that mesenchymal stem cell transplantation can contribute to spinal cord regeneration by:
- Replacement of nerve cells that have died as a result of the injury;
- Generate new supporting cells that will re-form the insulating nerve sheath (myelin) and act as a bridge across the injury to stimulate regrowth of the injured axons;
- Protect cells at the site of injury from further damage by releasing protective substances such as growth factors and absorbing toxins such as free radicals when introduced into the spinal cord shortly after injury.
- Preventing the spread of injury by suppressing the harmful inflammation that can occur after injury
In medical practice, it has been found that the secretory function of stem cells can initiate a self-renewal function in nerve tissue. Thus, stem cells are transplanted into the body and can therefore participate in the repair of nerve injury.
Purpose of treatment:
When transplanted into the injured spinal cord, stem cells can provide protective molecules/factors to tissues and help restore some of the circuits important to the network of nerves that carry information throughout the body. So, the goal of the treatment is to improve the patient's breathing, his or her defect control ability, his or her ability to move, and the sensitivity of the lower extremities.
DAMAGE TO ARTICULAR CARTILAGE
Cartilage structures and functions can be damaged. Such damage can result from a variety of causes, such as a bad fall or traumatic sports accident, previous knee injuries, or wear and tear over time. Immobilization for long periods can also result in cartilage damage. People with previous surgical interventions face more chances of articular cartilage damage due to altered joint mechanics. Articular cartilage damage can also be found in the shoulder, causing pain, discomfort and limited movement. Articular cartilage usually does not regenerate.
How can mesenchymal stem cells help?
Mesenchymal stem cells are emerging as an alternative to the use of differentiated chondrocytes. This has been achieved due to their potential to differentiate into several lineages such as osteoblasts, chondrocytes, myoblasts or adipocytes and their capacity for self-renewal, high plasticity and immunosuppressive and anti-inflammatory effects. They can be derived from various sources, such as bone marrow, periosteum, umbilical cord blood, dermis, muscle, infrapatellar fat pad, synovial membrane and adipose tissue.
Autologous cell implantation methods:
- Stromal Vascular Fraction (SVF)
SVF is derived from the patient's own adipose tissue. Adipose tissue is a rich and very convenient source of cells for therapeutic approaches in regenerative medicine. When fat is harvested for stem cells, the final product is called stromal vascular fraction. SVF contains a higher percentage of stromal elements. SVF cells are immediately injected into the target joint or into the connective tissue of the target joint.
- Autologous chondrocyte implantation (ACI)
In this method, mesenchymal stem cells are isolated from the patient's own cartilage tissue. Autologous chondrocyte implantation (ACI) is a relatively new, advanced procedure used to treat isolated articular cartilage defects of the knee. ACI has also been performed for defects of the patella (knee cap) and other joints. Autologous chondrocyte implantation is a two-stage surgical procedure.
The first procedure is performed arthroscopically in less than 30 minutes. The surgeon takes a small piece of articular cartilage from the knee. This cartilage biopsy is then sent to a lab where it is enzymatically processed to isolate chondrocytes, which are the body's cartilage-producing cells. Once these chondrocytes are obtained, they are expanded and sent back to the surgeon approximately 6 to 8 weeks later for implantation.
The second-stage surgery is an open procedure in which a small patch is sewn over the plastic cartilage defect. Chondrocytes that have been collected and expanded are injected under this patch, where they adhere to the patient's knee to form what is called hyaline-like cartilage, which resembles the joint's own cartilage. After implantation, there is a period of limited loading for up to 8 weeks. During this time, physical therapy is prescribed to increase range of motion and strengthen the musculature. Return to light sporting activities is usually allowed after approximately 6 months with return to full sporting activities between 9 and 12 months after the procedure. The overall success rate of ACI is approximately 85%.
When articular cartilage is damaged, stem cells are expected to immigrate and regenerate the damaged cartilage tissue.
OPTIC NEUROPATHY
Optic neuropathy refers to damage to the optic nerve due to some cause. Damage and death of these nerve cells or neurons results in the characteristic features of optic neuropathy. The main symptoms are:
- loss of vision
- the field of vision is narrowed
- vision defect
- hemianopsia, with the colors appearing finely washed in the affected eye
On medical examination, the optic nerve head can be visualized with an ophthalmoscope. A pale disc is characteristic of longstanding optic neuropathy. In many cases, only one eye is affected, and patients may not be aware of the loss of color vision until the doctor asks them to cover the healthy eye.
Reasons:
Optic neuropathy can be caused by any of the following:
- Ischemic optic neuropathy
- Optic neuritis
- Compressive optic neuropathy
- Infiltrative optic neuropathy
- Traumatic optic neuropathy
- Mitochondrial optic neuropathies
- Nutritional optic neuropathies
- Toxic optic neuropathies
- Hereditary optic neuropathies
How can stem cells contribute to optic neuropathy?
Stem cells can act as a source of new, healthy specialized cells and can provide a way to replace damaged cells in the eye. Mesenchymal stem cells can differentiate into photoreceptor cells and immune repair cells. The differentiated cells will first reconstitute the synaptic nucleoproteins of patients' autologous cells. And finally they will play a positive role in the treatment of lesions in retinal pigment cells and optic nerves. Once positioned in the lesional area, they will differentiate into functional optic neurons, oligodendrocytes and astrocytes. This will increase the number and recovery of optic nerve cells as well as the regulation of optic nerve to retinal and optic photoreceptor cells.
For patients who suffer from optic nerve injuries or atrophy, such treatment will effectively replenish missing cells and increase fiber recovery. All of this will lead to achieving the goal of improving patients' vision, visual field and color discrimination ability.
CEREBRAL PALSY
Cerebral palsy (CP) is a developmental disorder of the nervous system. Usually, cerebral palsy is referred to as a central movement disorder that is caused by a non-progressive brain injury or encephalodysplasia during pregnancy, during childbirth, or after birth up to about one month for many reasons. The incidence of cerebral palsy is about 1.2-2.5%.
Pathogenesis
There are many causes of cerebral palsy, such as:
- genetic diseases
- infectious diseases
- cerebral dysplasia
- cerebral ischemia and hypoxia
- brain damage
- cerebral hemorrhage, etc.
Although prematurity and intrauterine growth retardation are not the direct causes of cerebral palsy, they are still the most important high-risk factors.
Clinical characteristic
Symptoms of patients with cerebral palsy in infancy usually show abnormal body posture and slowed movement, such as neonatal hypoxic ischemic encephalopathy and low muscle tone in the early periods of infancy, then developing into hypermyotonia. The disturbance of balance can be detected once the infant can sit or stand. Secondary changes, such as joint contracture and spinal deformity, are progressive. Secondary changes may also include epilepsy, mental retardation, behavioural disorders and sensory impairment.
What is stem cell transplantation for children with cerebral palsy?
During a stem cell transplant, a doctor injects stem cells into the child either intravenously or through a lumbar puncture in the spinal cord area. The hope is that the stem cells will develop into nerve cells and into an area where the cells can develop connections with other cells. This is necessary for the treatment to be useful. In addition, once the cells connect with other cells, they must be able to transmit messages to the muscles that control movement for the treatment to be successful.
Physical therapy is almost always recommended after stem cell therapy. Medical experts state that while it is difficult to know whether stem cell therapy or physical therapy worked best for treatment, the combination of the two gives the child the best chance of success.
Improvement:
Most cerebral palsy patients who have been treated using a combination of stem cell therapy and rehabilitation show visible signs of improvement:
- recovery of motor development and coordination
- regaining vision
- improving mental retardation
- increase muscle strength
- decrease in muscle tone spasticity, etc.
However, when discussing improvements, it is important to remember that they can vary considerably from patient to patient due to many factors, such as:
- patient's medical course
- physical condition
- burden
- age, etc.
Therefore, improvement cannot be guaranteed.
MUSCULAR DYSTROPHY
Progressive muscular dystrophy, PMD, is a group of inherited, progressive degenerative diseases of skeletal muscle.
The main clinical manifestations are slowly progressive muscular dystrophy and muscle weakness. Cardiac muscle atrophy and skeletal muscle atrophy occurs in some species. Different gene defects result in different timing of onset of symptoms.
According to various clinical manifestations or gene defects, the clinical classifications are congenital muscular dystrophy, Duchenne muscular dystrophy, Becker muscular dystrophy, limb-girdle muscular dystrophy, and so on. Duchenne muscular dystrophy and Becker muscular dystrophy are the most common clinical types. From the name, we can understand that muscular dystrophy is progressive, but the speed of disease development is different.
The majority of patients with progressive muscular dystrophy have a poor prognosis; muscle paralysis and atrophy can lead to patient disability and/or organ failure. Conventional medical treatment includes symptomatic treatment, supportive care and education with rehabilitation. But these treatments cannot stop the progression of the disease as these conventional drug treatments can neither reduce the rate of degeneration of muscle fiber damage nor increase the number of muscle fibers. So stem cell transplantation may be more effective for patients with muscular dystrophy if administered with conventional drugs as a reinforcing treatment.
Treatment procedure:
Patients receive umbilical cord-derived mesenchymal stem cell transplants via intramuscular (for affected muscles) injection and intravenous injections.
Restorative treatment includes physiotherapy to prevent and restore deformities and contractures.
More recently, it has been shown that mesenchymal stem cells can differentiate the muscle fiber cell because these cells can secrete dystrophin, which has complement. It can effectively reduce the rate of muscle fiber damage, reduce creatine phosphokinase, stop the progression of muscle dystrophy, and restore strength in parts of the muscle.
Clinical trials are ongoing for stem cell treatments for incurable diseases such as Spinocerebellar/Frederich ataxia, Hypoxic encephalopathy, spinal cord injuries, cerebral palsy, Duchenne muscular dystrophy and others. Regenerative medicine is developing at an extraordinary pace. Particular emphasis is being placed on cell therapies that have the potential to produce dramatic clinical benefits and address important unmet medical needs. Regenerative medicine is not just a future hope, it is a reality today.
The specialists: